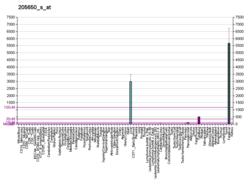
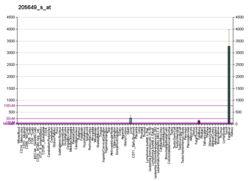
Fibrinogen alpha chain
| FGA | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | FGA, Fib2, fibrinogen alpha chain | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 134820; MGI: 1316726; HomoloGene: 428; GeneCards: FGA; OMA:FGA - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Fibrinogen alpha chain is a protein that in humans is encoded by the FGA gene.
Function
The protein encoded by this gene is the alpha component of fibrinogen, a blood-borne glycoprotein composed of three pairs of nonidentical polypeptide chains. Following vascular injury, fibrinogen is cleaved by thrombin to form fibrin, which is the most abundant component of blood clots. In addition, various cleavage products of fibrinogen and fibrin regulate cell adhesion and spreading, display vasoconstrictor and chemotactic activities, and are mitogens for several cell types. Mutations in this gene lead to several disorders, including dysfibrinogenemia, hypofibrinogenemia, afibrinogenemia, and renal amyloidosis. Alternative splicing results in two isoforms that vary in the carboxy-terminus.[5]
Interactions
Fibrinogen alpha chain has been shown to interact with tissue plasminogen activator.[6][7]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000171560 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000028001 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Entrez Gene: FGA fibrinogen alpha chain".
- ^ Tsurupa G, Medved L (Jan 2001). "Identification and characterization of novel tPA- and plasminogen-binding sites within fibrin(ogen) alpha C-domains". Biochemistry. 40 (3): 801–808. doi:10.1021/bi001789t. PMID 11170397.
- ^ Ichinose A, Takio K, Fujikawa K (Jul 1986). "Localization of the binding site of tissue-type plasminogen activator to fibrin". The Journal of Clinical Investigation. 78 (1): 163–169. doi:10.1172/JCI112546. PMC 329545. PMID 3088041.
Further reading
- Doolittle RF (1984). "Fibrinogen and fibrin". Annual Review of Biochemistry. 53: 195–229. doi:10.1146/annurev.bi.53.070184.001211. PMID 6383194.
- Galanakis DK (1994). "Inherited dysfibrinogenemia: emerging abnormal structure associations with pathologic and nonpathologic dysfunctions". Seminars in Thrombosis and Hemostasis. 19 (4): 386–395. doi:10.1055/s-2007-993290. PMID 8140431. S2CID 739367.
- Herrick S, Blanc-Brude O, Gray A, Laurent G (Jul 1999). "Fibrinogen". The International Journal of Biochemistry & Cell Biology. 31 (7): 741–746. doi:10.1016/S1357-2725(99)00032-1. PMID 10467729.
- Bennett JS (2001). "Platelet-fibrinogen interactions". Annals of the New York Academy of Sciences. 936 (1): 340–354. Bibcode:2001NYASA.936..340B. doi:10.1111/j.1749-6632.2001.tb03521.x. PMID 11460491. S2CID 25431334.
- Redman CM, Xia H (2001). "Fibrinogen biosynthesis. Assembly, intracellular degradation, and association with lipid synthesis and secretion". Annals of the New York Academy of Sciences. 936: 480–495. doi:10.1111/j.1749-6632.2001.tb03535.x. PMID 11460506. S2CID 31741202.
- Matsuda M, Sugo T (August 2002). "Structure and function of human fibrinogen inferred from dysfibrinogens". International Journal of Hematology. 76 (Suppl 1): 352–60. doi:10.1007/bf03165284. PMID 12430881. S2CID 11165476.
- Everse SJ (August 2002). "New insights into fibrin (ogen) structure and function". Vox Sanguinis. 83 (Suppl 1): 375–82. doi:10.1111/j.1423-0410.2002.tb05338.x. PMID 12617173. S2CID 21813767.
- Scott EM, Ariëns RA, Grant PJ (September 2004). "Genetic and environmental determinants of fibrin structure and function: relevance to clinical disease". Arteriosclerosis, Thrombosis, and Vascular Biology. 24 (9): 1558–1566. doi:10.1161/01.ATV.0000136649.83297.bf. PMID 15217804. S2CID 21298700.
- Lord ST (May 2007). "Fibrinogen and fibrin: scaffold proteins in hemostasis". Current Opinion in Hematology. 14 (3): 236–241. doi:10.1097/MOH.0b013e3280dce58c. PMID 17414213. S2CID 31315177.
- Cottrell BA, Strong DD, Watt KW, Doolittle RF (November 1979). "Amino acid sequence studies on the alpha chain of human fibrinogen. Exact location of cross-linking acceptor sites". Biochemistry. 18 (24): 5405–5410. doi:10.1021/bi00591a023. PMID 518845.
- Watt KW, Cottrell BA, Strong DD, Doolittle RF (November 1979). "Amino acid sequence studies on the alpha chain of human fibrinogen. Overlapping sequences providing the complete sequence". Biochemistry. 18 (24): 5410–5416. doi:10.1021/bi00591a024. PMID 518846.
- Fretto LJ, Ferguson EW, Steinman HM, McKee PA (Apr 1978). "Localization of the alpha-chain cross-link acceptor sites of human fibrin". The Journal of Biological Chemistry. 253 (7): 2184–95. doi:10.1016/S0021-9258(17)38057-2. PMID 632262.
- Blombäck B, Hessel B, Hogg D (May 1976). "Disulfide bridges in nh2 -terminal part of human fibrinogen". Thrombosis Research. 8 (5): 639–658. doi:10.1016/0049-3848(76)90245-0. PMID 936108.
- Koopman J, Haverkate F, Grimbergen J, Egbring R, Lord ST (Oct 1992). "Fibrinogen Marburg: a homozygous case of dysfibrinogenemia, lacking amino acids A alpha 461-610 (Lys 461 AAA→stop TAA)". Blood. 80 (8): 1972–9. doi:10.1182/blood.V80.8.1972.1972. PMID 1391954.
- Fu Y, Weissbach L, Plant PW, Oddoux C, Cao Y, Liang TJ, Roy SN, Redman CM, Grieninger G (December 1992). "Carboxy-terminal-extended variant of the human fibrinogen alpha subunit: a novel exon conferring marked homology to beta and gamma subunits". Biochemistry. 31 (48): 11968–11972. doi:10.1021/bi00163a002. PMID 1457396.
- Martin PD, Robertson W, Turk D, Huber R, Bode W, Edwards BF (Apr 1992). "The structure of residues 7-16 of the A alpha-chain of human fibrinogen bound to bovine thrombin at 2.3-A resolution". The Journal of Biological Chemistry. 267 (11): 7911–20. doi:10.1016/S0021-9258(18)42599-9. PMID 1560020.
- Stubbs MT, Oschkinat H, Mayr I, Huber R, Angliker H, Stone SR, Bode W (May 1992). "The interaction of thrombin with fibrinogen. A structural basis for its specificity". European Journal of Biochemistry. 206 (1): 187–195. doi:10.1111/j.1432-1033.1992.tb16916.x. PMID 1587268.
- Maekawa H, Yamazumi K, Muramatsu S, Kaneko M, Hirata H, Takahashi N, Arocha-Piñango CL, Rodriguez S, Nagy H, Perez-Requejo JL (Jul 1992). "Fibrinogen Lima: a homozygous dysfibrinogen with an A alpha-arginine-141 to serine substitution associated with extra N-glycosylation at A alpha-asparagine-139. Impaired fibrin gel formation but normal fibrin-facilitated plasminogen activation catalyzed by tissue-type plasminogen activator". The Journal of Clinical Investigation. 90 (1): 67–76. doi:10.1172/JCI115857. PMC 443064. PMID 1634621.
- Maekawa H, Yamazumi K, Muramatsu S, Kaneko M, Hirata H, Takahashi N, de Bosch NB, Carvajal Z, Ojeda A, Arocha-Piñango CL (Jun 1991). "An A alpha Ser-434 to N-glycosylated Asn substitution in a dysfibrinogen, fibrinogen Caracas II, characterized by impaired fibrin gel formation". The Journal of Biological Chemistry. 266 (18): 11575–81. doi:10.1016/S0021-9258(18)98995-7. PMID 1675636.
- Wu C, Chung AE (Oct 1991). "Potential role of entactin in hemostasis. Specific interaction of entactin with fibrinogen A alpha and B beta chains". The Journal of Biological Chemistry. 266 (28): 18802–7. doi:10.1016/S0021-9258(18)55134-6. PMID 1680863.
- v
- t
- e
-
 1fza: CRYSTAL STRUCTURE OF FIBRINOGEN FRAGMENT D
1fza: CRYSTAL STRUCTURE OF FIBRINOGEN FRAGMENT D -
 1fzb: CRYSTAL STRUCTURE OF CROSSLINKED FRAGMENT D
1fzb: CRYSTAL STRUCTURE OF CROSSLINKED FRAGMENT D -
 1fzc: CRYSTAL STRUCTURE OF FRAGMENT DOUBLE-D FROM HUMAN FIBRIN WITH TWO DIFFERENT BOUND LIGANDS
1fzc: CRYSTAL STRUCTURE OF FRAGMENT DOUBLE-D FROM HUMAN FIBRIN WITH TWO DIFFERENT BOUND LIGANDS -
 1fzd: STRUCTURE OF RECOMBINANT ALPHAEC DOMAIN FROM HUMAN FIBRINOGEN-420
1fzd: STRUCTURE OF RECOMBINANT ALPHAEC DOMAIN FROM HUMAN FIBRINOGEN-420 -
 1fze: CRYSTAL STRUCTURE OF FRAGMENT DOUBLE-D FROM HUMAN FIBRIN
1fze: CRYSTAL STRUCTURE OF FRAGMENT DOUBLE-D FROM HUMAN FIBRIN -
 1fzf: CRYSTAL STRUCTURE OF FRAGMENT DOUBLE-D FROM HUMAN FIBRIN WITH THE PEPTIDE LIGAND GLY-HIS-ARG-PRO-AMIDE
1fzf: CRYSTAL STRUCTURE OF FRAGMENT DOUBLE-D FROM HUMAN FIBRIN WITH THE PEPTIDE LIGAND GLY-HIS-ARG-PRO-AMIDE -
 1fzg: CRYSTAL STRUCTURE OF FRAGMENT D FROM HUMAN FIBRINOGEN WITH THE PEPTIDE LIGAND GLY-HIS-ARG-PRO-AMIDE
1fzg: CRYSTAL STRUCTURE OF FRAGMENT D FROM HUMAN FIBRINOGEN WITH THE PEPTIDE LIGAND GLY-HIS-ARG-PRO-AMIDE -
 1lt9: Crystal Structure of Recombinant Human Fibrinogen Fragment D
1lt9: Crystal Structure of Recombinant Human Fibrinogen Fragment D -
 1ltj: Crystal Structure of Recombinant Human Fibrinogen Fragment D with the Peptide Ligands Gly-Pro-Arg-Pro-Amide and Gly-His-Arg-Pro-Amide
1ltj: Crystal Structure of Recombinant Human Fibrinogen Fragment D with the Peptide Ligands Gly-Pro-Arg-Pro-Amide and Gly-His-Arg-Pro-Amide -
 1n86: Crystal structure of human D-dimer from cross-linked fibrin complexed with GPR and GHRPLDK peptide ligands.
1n86: Crystal structure of human D-dimer from cross-linked fibrin complexed with GPR and GHRPLDK peptide ligands. -
 1n8e: Fragment Double-D from Human Fibrin
1n8e: Fragment Double-D from Human Fibrin -
 1re3: Crystal Structure of Fragment D of BbetaD398A Fibrinogen with the Peptide Ligand Gly-His-Arg-Pro-Amide
1re3: Crystal Structure of Fragment D of BbetaD398A Fibrinogen with the Peptide Ligand Gly-His-Arg-Pro-Amide -
 1re4: Crystal Structure of Fragment D of BbetaD398A Fibrinogen
1re4: Crystal Structure of Fragment D of BbetaD398A Fibrinogen -
 1rf0: Crystal Structure of Fragment D of gammaE132A Fibrinogen
1rf0: Crystal Structure of Fragment D of gammaE132A Fibrinogen -
 1rf1: Crystal Structure of Fragment D of gammaE132A Fibrinogen with the Peptide Ligand Gly-His-Arg-Pro-amide
1rf1: Crystal Structure of Fragment D of gammaE132A Fibrinogen with the Peptide Ligand Gly-His-Arg-Pro-amide -
 2a45: Crystal structure of the complex between thrombin and the central ""E"" region of fibrin
2a45: Crystal structure of the complex between thrombin and the central ""E"" region of fibrin -
 2ffd: Fibrinogen Fragment D with ""A"" knob peptide mimic GPRVVE
2ffd: Fibrinogen Fragment D with ""A"" knob peptide mimic GPRVVE -
 2h43: Crystal Structure of Human Fragment D Complexed with Ala-His-Arg-Pro-amide
2h43: Crystal Structure of Human Fragment D Complexed with Ala-His-Arg-Pro-amide -
 2hod: Crystal Structure of Fragment D from Human Fibrinogen Complexed with Gly-hydroxyPro-Arg-Pro-amide
2hod: Crystal Structure of Fragment D from Human Fibrinogen Complexed with Gly-hydroxyPro-Arg-Pro-amide -
 2hpc: Crystal structure of fragment D from Human Fibrinogen Complexed with Gly-Pro-Arg-Pro-amide.
2hpc: Crystal structure of fragment D from Human Fibrinogen Complexed with Gly-Pro-Arg-Pro-amide. -
 2oyh: Crystal Structure of Fragment D of gammaD298,301A Fibrinogen with the Peptide Ligand Gly-His-Arg-Pro-Amide
2oyh: Crystal Structure of Fragment D of gammaD298,301A Fibrinogen with the Peptide Ligand Gly-His-Arg-Pro-Amide -
 2oyi: Crystal Structure of Fragment D of gammaD298,301A Fibrinogen with the Peptide Ligand Gly-Pro-Arg-Pro-Amide
2oyi: Crystal Structure of Fragment D of gammaD298,301A Fibrinogen with the Peptide Ligand Gly-Pro-Arg-Pro-Amide