Protein-coding gene in the species Homo sapiens
| MAP2K5 |
|---|
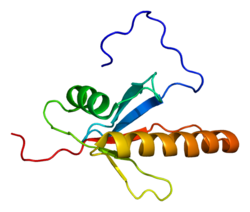 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
2NPT, 2O2V, 4IC7 |
|
|
| Identifiers |
|---|
| Aliases | MAP2K5, HsT17454, MAPKK5, MEK5, PRKMK5, mitogen-activated protein kinase kinase 5 |
|---|
| External IDs | OMIM: 602520; MGI: 1346345; HomoloGene: 115933; GeneCards: MAP2K5; OMA:MAP2K5 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 15 (human)[1] |
|---|
| | Band | 15q23 | Start | 67,542,703 bp[1] |
|---|
| End | 67,807,117 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 9 (mouse)[2] |
|---|
| | Band | 9|9 B- C | Start | 63,071,050 bp[2] |
|---|
| End | 63,285,184 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - right testis
- left testis
- parotid gland
- gonad
- right frontal lobe
- Achilles tendon
- gastrocnemius muscle
- ventricular zone
- Brodmann area 9
- prefrontal cortex
|
| | Top expressed in | - internal carotid artery
- external carotid artery
- motor neuron
- Rostral migratory stream
- spermatocyte
- CA3 field
- right kidney
- seminiferous tubule
- primitive streak
- left lobe of liver
|
| | More reference expression data |
|
|---|
| BioGPS | 
 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - transferase activity
- protein kinase activity
- nucleotide binding
- metal ion binding
- kinase activity
- protein binding
- protein tyrosine kinase activity
- ATP binding
- protein serine/threonine kinase activity
- MAP kinase kinase activity
| | Cellular component | - cytoplasm
- cytosol
- spindle
- nucleus
- intracellular anatomical structure
| | Biological process | - negative regulation of chemokine (C-X-C motif) ligand 2 production
- negative regulation of cysteine-type endopeptidase activity involved in apoptotic process
- phosphorylation
- positive regulation of epithelial cell proliferation
- cellular response to laminar fluid shear stress
- cellular response to growth factor stimulus
- negative regulation of transcription by RNA polymerase II
- negative regulation of response to cytokine stimulus
- MAPK cascade
- ERK5 cascade
- negative regulation of cell migration involved in sprouting angiogenesis
- protein phosphorylation
- heart development
- positive regulation of cell growth
- peptidyl-tyrosine phosphorylation
- negative regulation of heterotypic cell-cell adhesion
- negative regulation of extrinsic apoptotic signaling pathway in absence of ligand
- negative regulation of NF-kappaB transcription factor activity
- signal transduction
- positive regulation of transcription by RNA polymerase II
- regulation of mitotic cell cycle
- stress-activated protein kinase signaling cascade
- activation of protein kinase activity
- regulation of apoptotic process
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_001206804
NM_002757
NM_145160
NM_145161
NM_145162 |
| |
|---|
NM_011840
NM_001364492
NM_001364493
NM_001364494 |
|
|---|
| RefSeq (protein) | |
|---|
NP_001193733
NP_002748
NP_660143 |
| |
|---|
NP_035970
NP_001351421
NP_001351422
NP_001351423 |
|
|---|
| Location (UCSC) | Chr 15: 67.54 – 67.81 Mb | Chr 9: 63.07 – 63.29 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Dual specificity mitogen-activated protein kinase kinase 5 is an enzyme that in humans is encoded by the MAP2K5 gene.[5][6]
Function
The protein encoded by this gene is a dual specificity protein kinase that belongs to the MAP kinase kinase family. This kinase specifically interacts with and activates MAPK7/ERK5. This kinase itself can be phosphorylated and activated by MAP3K3/MEKK3, as well as by atypical protein kinase C isoforms (aPKCs). The signal cascade mediated by this kinase is involved in growth factor stimulated cell proliferation and muscle cell differentiation. Four alternatively spliced transcript variants of this gene encoding distinct isoforms have been described.[6]
Upstream
This kinase itself can be phosphorylated and activated by MAP3K3/MEKK3, as well as by atypical protein kinase C isoforms (aPKCs).
Downstream
This kinase specifically interacts with and activates MAPK7/ERK5.
Interactions
MAP2K5 has been shown to interact with MAPK7,[5] MAP3K2,[7] Protein kinase Mζ[8] and MAP3K3.[7][9]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000137764 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000058444 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Zhou G, Bao ZQ, Dixon JE (May 1995). "Components of a new human protein kinase signal transduction pathway". The Journal of Biological Chemistry. 270 (21): 12665–9. doi:10.1074/jbc.270.21.12665. PMID 7759517.
- ^ a b "Entrez Gene: MAP2K5 mitogen-activated protein kinase kinase 5".
- ^ a b Sun W, Kesavan K, Schaefer BC, Garrington TP, Ware M, Johnson NL, Gelfand EW, Johnson GL (Feb 2001). "MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway". The Journal of Biological Chemistry. 276 (7): 5093–100. doi:10.1074/jbc.M003719200. PMID 11073940.
- ^ Diaz-Meco MT, Moscat J (Feb 2001). "MEK5, a new target of the atypical protein kinase C isoforms in mitogenic signaling". Molecular and Cellular Biology. 21 (4): 1218–27. doi:10.1128/MCB.21.4.1218-1227.2001. PMC 99575. PMID 11158308.
- ^ Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G (Feb 2004). "A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway". Nature Cell Biology. 6 (2): 97–105. doi:10.1038/ncb1086. PMID 14743216. S2CID 11683986.
Further reading
- English JM, Vanderbilt CA, Xu S, Marcus S, Cobb MH (Dec 1995). "Isolation of MEK5 and differential expression of alternatively spliced forms". The Journal of Biological Chemistry. 270 (48): 28897–902. doi:10.1074/jbc.270.48.28897. PMID 7499418.
- Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD (Dec 1997). "BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C". The EMBO Journal. 16 (23): 7054–66. doi:10.1093/emboj/16.23.7054. PMC 1170308. PMID 9384584.
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD (Oct 1998). "Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor". Nature. 395 (6703): 713–6. Bibcode:1998Natur.395..713K. doi:10.1038/27234. PMID 9790194. S2CID 204997780.
- English JM, Pearson G, Hockenberry T, Shivakumar L, White MA, Cobb MH (Oct 1999). "Contribution of the ERK5/MEK5 pathway to Ras/Raf signaling and growth control". The Journal of Biological Chemistry. 274 (44): 31588–92. doi:10.1074/jbc.274.44.31588. PMID 10531364.
- Chao TH, Hayashi M, Tapping RI, Kato Y, Lee JD (Dec 1999). "MEKK3 directly regulates MEK5 activity as part of the big mitogen-activated protein kinase 1 (BMK1) signaling pathway". The Journal of Biological Chemistry. 274 (51): 36035–8. doi:10.1074/jbc.274.51.36035. PMID 10593883.
- Sun W, Kesavan K, Schaefer BC, Garrington TP, Ware M, Johnson NL, Gelfand EW, Johnson GL (Feb 2001). "MEKK2 associates with the adapter protein Lad/RIBP and regulates the MEK5-BMK1/ERK5 pathway". The Journal of Biological Chemistry. 276 (7): 5093–100. doi:10.1074/jbc.M003719200. PMID 11073940.
- Diaz-Meco MT, Moscat J (Feb 2001). "MEK5, a new target of the atypical protein kinase C isoforms in mitogenic signaling". Molecular and Cellular Biology. 21 (4): 1218–27. doi:10.1128/MCB.21.4.1218-1227.2001. PMC 99575. PMID 11158308.
- Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN (Jun 2001). "Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy". The EMBO Journal. 20 (11): 2757–67. doi:10.1093/emboj/20.11.2757. PMC 125475. PMID 11387209.
- Dinev D, Jordan BW, Neufeld B, Lee JD, Lindemann D, Rapp UR, Ludwig S (Sep 2001). "Extracellular signal regulated kinase 5 (ERK5) is required for the differentiation of muscle cells". EMBO Reports. 2 (9): 829–34. doi:10.1093/embo-reports/kve177. PMC 1084032. PMID 11520859.
- Weldon CB, Scandurro AB, Rolfe KW, Clayton JL, Elliott S, Butler NN, Melnik LI, Alam J, McLachlan JA, Jaffe BM, Beckman BS, Burow ME (Aug 2002). "Identification of mitogen-activated protein kinase kinase as a chemoresistant pathway in MCF-7 cells by using gene expression microarray". Surgery. 132 (2): 293–301. doi:10.1067/msy.2002.125389. PMID 12219026.
- Mehta PB, Jenkins BL, McCarthy L, Thilak L, Robson CN, Neal DE, Leung HY (Mar 2003). "MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion". Oncogene. 22 (9): 1381–9. doi:10.1038/sj.onc.1206154. PMID 12618764.
- Mody N, Campbell DG, Morrice N, Peggie M, Cohen P (Jun 2003). "An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro". The Biochemical Journal. 372 (Pt 2): 567–75. doi:10.1042/BJ20030193. PMC 1223423. PMID 12628002.
- Huang J, Tu Z, Lee FS (Apr 2003). "Mutations in protein kinase subdomain X differentially affect MEKK2 and MEKK1 activity". Biochemical and Biophysical Research Communications. 303 (2): 532–40. doi:10.1016/S0006-291X(03)00387-5. PMID 12659851.
- Lamark T, Perander M, Outzen H, Kristiansen K, Øvervatn A, Michaelsen E, Bjørkøy G, Johansen T (Sep 2003). "Interaction codes within the family of mammalian Phox and Bem1p domain-containing proteins". The Journal of Biological Chemistry. 278 (36): 34568–81. doi:10.1074/jbc.M303221200. PMID 12813044.
- Cameron SJ, Abe J, Malik S, Che W, Yang J (Jan 2004). "Differential role of MEK5alpha and MEK5beta in BMK1/ERK5 activation". The Journal of Biological Chemistry. 279 (2): 1506–12. doi:10.1074/jbc.M308755200. PMID 14583600.
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G (Feb 2004). "A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway". Nature Cell Biology. 6 (2): 97–105. doi:10.1038/ncb1086. PMID 14743216. S2CID 11683986.
- Raviv Z, Kalie E, Seger R (Apr 2004). "MEK5 and ERK5 are localized in the nuclei of resting as well as stimulated cells, while MEKK2 translocates from the cytosol to the nucleus upon stimulation". Journal of Cell Science. 117 (Pt 9): 1773–84. doi:10.1242/jcs.01040. PMID 15075238.
PDB gallery
-
1wi0: Solution structure of the PB1 domain of mouse mitogen activated protein kinase kinase 5 (MAP2K5) -
2npt: Crystal Structure of the complex of human mitogen activated protein kinase kinase 5 phox domain (MAP2K5-phox) with human mitogen activated protein kinase kinase kinase 2 phox domain (MAP3K2-phox) -
2o2v: Crystal Structure of the Complex of Human Mitogen Activated Protein Kinase Kinase 5 Phox Domain (MAP2K5-phox) with Human Mitogen Activated Protein Kinase Kinase Kinase 3 (MAP3K3B-phox) |
|
|---|
| Activity | |
|---|
| Regulation | |
|---|
| Classification | |
|---|
| Kinetics | |
|---|
| Types | |
|---|
Portal: Biology
Biology
 | This article on a gene on human chromosome 15 is a stub. You can help Wikipedia by expanding it. |

 1wi0: Solution structure of the PB1 domain of mouse mitogen activated protein kinase kinase 5 (MAP2K5)
1wi0: Solution structure of the PB1 domain of mouse mitogen activated protein kinase kinase 5 (MAP2K5) 2npt: Crystal Structure of the complex of human mitogen activated protein kinase kinase 5 phox domain (MAP2K5-phox) with human mitogen activated protein kinase kinase kinase 2 phox domain (MAP3K2-phox)
2npt: Crystal Structure of the complex of human mitogen activated protein kinase kinase 5 phox domain (MAP2K5-phox) with human mitogen activated protein kinase kinase kinase 2 phox domain (MAP3K2-phox) 2o2v: Crystal Structure of the Complex of Human Mitogen Activated Protein Kinase Kinase 5 Phox Domain (MAP2K5-phox) with Human Mitogen Activated Protein Kinase Kinase Kinase 3 (MAP3K3B-phox)
2o2v: Crystal Structure of the Complex of Human Mitogen Activated Protein Kinase Kinase 5 Phox Domain (MAP2K5-phox) with Human Mitogen Activated Protein Kinase Kinase Kinase 3 (MAP3K3B-phox)























