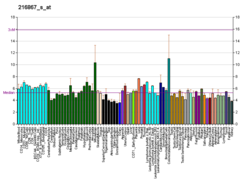
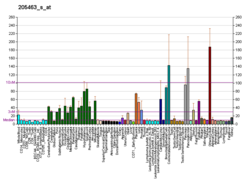
PDGFA
| PDGFA | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | PDGFA, PDGF-A, PDGF1, platelet derived growth factor subunit A | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 173430 MGI: 97527 HomoloGene: 32055 GeneCards: PDGFA | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Platelet-derived growth factor subunit A is a protein that in humans is encoded by the PDGFA gene.[5][6]
The protein encoded by this gene is a member of the platelet-derived growth factor family. The four members of this family are mitogenic factors for cells of mesenchymal origin and are characterized by a motif of eight cysteines. This gene product can exist either as a homodimer or as a heterodimer with the platelet-derived growth factor beta polypeptide, where the dimers are connected by disulfide bonds. Studies using knockout mice have shown cellular defects in oligodendrocytes, alveolar smooth muscle cells, and Leydig cells in the testis; knockout mice die either as embryos or shortly after birth. Two splice variants have been identified for this gene.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000197461 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000025856 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Stenman G, Rorsman F, Huebner K, Betsholtz C (Sep 1992). "The human platelet-derived growth factor alpha chain (PDGFA) gene maps to chromosome 7p22". Cytogenet Cell Genet. 60 (3–4): 206–7. doi:10.1159/000133337. PMID 1505216.
- ^ Matsui T, Heidaran M, Miki T, Popescu N, La Rochelle W, Kraus M, Pierce J, Aaronson S (Mar 1989). "Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes". Science. 243 (4892): 800–4. Bibcode:1989Sci...243..800M. doi:10.1126/science.2536956. PMID 2536956.
- ^ "Entrez Gene: PDGFA platelet-derived growth factor alpha polypeptide".
Further reading
- Nath K, Gogi R, Khan AA, Hameed S (1978). "Vascular hamartoma and vascular tumours of orbit". Indian Journal of Ophthalmology. 25 (1): 18–23. PMID 612586.
- Ishibashi T, Bottaro DP, Chan A, et al. (1993). "Expression cloning of a human dual-specificity phosphatase". Proc. Natl. Acad. Sci. U.S.A. 89 (24): 12170–4. doi:10.1073/pnas.89.24.12170. PMC 50720. PMID 1281549.
- Raines EW, Lane TF, Iruela-Arispe ML, et al. (1992). "The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors". Proc. Natl. Acad. Sci. U.S.A. 89 (4): 1281–5. Bibcode:1992PNAS...89.1281R. doi:10.1073/pnas.89.4.1281. PMC 48433. PMID 1311092.
- Andersson M, Ostman A, Bäckström G, et al. (1992). "Assignment of interchain disulfide bonds in platelet-derived growth factor (PDGF) and evidence for agonist activity of monomeric PDGF". J. Biol. Chem. 267 (16): 11260–6. doi:10.1016/S0021-9258(19)49905-5. PMID 1317862.
- Rorsman F, Leveen P, Betsholtz C (1993). "Platelet-derived growth factor (PDGF) A-chain mRNA heterogeneity generated by the use of alternative promoters and alternative polyadenylation sites". Growth Factors. 7 (3): 241–51. doi:10.3109/08977199209046928. PMID 1360804.
- Wang Z, Lin XH, Qiu QQ, Deuel TF (1992). "Modulation of transcription of the platelet-derived growth factor A-chain gene by a promoter region sensitive to S1 nuclease". J. Biol. Chem. 267 (24): 17022–31. doi:10.1016/S0021-9258(18)41887-X. PMID 1512241.
- Bonthron D, Collins T, Grzeschik KH, et al. (1992). "Platelet-derived growth factor A chain: confirmation of localization of PDGFA to chromosome 7p22 and description of an unusual minisatellite". Genomics. 13 (2): 257–63. doi:10.1016/0888-7543(92)90240-S. PMID 1612586.
- Takimoto Y, Wang ZY, Kobler K, Deuel TF (1991). "Promoter region of the human platelet-derived growth factor A-chain gene". Proc. Natl. Acad. Sci. U.S.A. 88 (5): 1686–90. Bibcode:1991PNAS...88.1686T. doi:10.1073/pnas.88.5.1686. PMC 51089. PMID 1848007.
- Fabisiak JP, Evans JN, Absher M, et al. (1991). "Expression of platelet-derived growth factor A-chain (PDGF-A) mRNA in lung tissue and cells. Complex regulation during remodeling". Chest. 99 (3 Suppl): 52S–54S. doi:10.1378/chest.99.3_Supplement.52S. PMID 1997274.
- Mendoza AE, Young R, Orkin SH, Collins T (1990). "Increased platelet-derived growth factor A-chain expression in human uterine smooth muscle cells during the physiologic hypertrophy of pregnancy". Proc. Natl. Acad. Sci. U.S.A. 87 (6): 2177–81. Bibcode:1990PNAS...87.2177M. doi:10.1073/pnas.87.6.2177. PMC 53649. PMID 2315311.
- Claesson-Welsh L, Eriksson A, Westermark B, Heldin CH (1989). "cDNA cloning and expression of the human A-type platelet-derived growth factor (PDGF) receptor establishes structural similarity to the B-type PDGF receptor". Proc. Natl. Acad. Sci. U.S.A. 86 (13): 4917–21. Bibcode:1989PNAS...86.4917C. doi:10.1073/pnas.86.13.4917. PMC 297526. PMID 2544881.
- Rorsman F, Bywater M, Knott TJ, et al. (1988). "Structural characterization of the human platelet-derived growth factor A-chain cDNA and gene: alternative exon usage predicts two different precursor proteins". Mol. Cell. Biol. 8 (2): 571–7. doi:10.1128/MCB.8.2.571. PMC 363182. PMID 2832727.
- Beckmann MP, Betsholtz C, Heldin CH, et al. (1988). "Comparison of biological properties and transforming potential of human PDGF-A and PDGF-B chains". Science. 241 (4871): 1346–9. Bibcode:1988Sci...241.1346B. doi:10.1126/science.2842868. PMID 2842868.
- Bonthron DT, Morton CC, Orkin SH, Collins T (1988). "Platelet-derived growth factor A chain: gene structure, chromosomal location, and basis for alternative mRNA splicing". Proc. Natl. Acad. Sci. U.S.A. 85 (5): 1492–6. Bibcode:1988PNAS...85.1492B. doi:10.1073/pnas.85.5.1492. PMC 279797. PMID 3422746.
- Tong BD, Auer DE, Jaye M, et al. (1987). "cDNA clones reveal differences between human glial and endothelial cell platelet-derived growth factor A-chains". Nature. 328 (6131): 619–21. doi:10.1038/328619a0. PMID 3614363. S2CID 4362771.
- Collins T, Bonthron DT, Orkin SH (1987). "Alternative RNA splicing affects function of encoded platelet-derived growth factor A chain". Nature. 328 (6131): 621–4. doi:10.1038/328621a0. PMID 3614364. S2CID 4268696.
- Hoppe J, Schumacher L, Eichner W, Weich HA (1987). "The long 3'-untranslated regions of the PDGF-A and -B mRNAs are only distantly related". FEBS Lett. 223 (2): 243–6. doi:10.1016/0014-5793(87)80297-1. PMID 3666150. S2CID 46032767.
- Betsholtz C, Johnsson A, Heldin CH, et al. (1986). "cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines". Nature. 320 (6064): 695–9. Bibcode:1986Natur.320..695B. doi:10.1038/320695a0. PMID 3754619. S2CID 4281536.
- Takimoto Y, Li WY, Wang ZY, et al. (1993). "Nucleotide sequence of the 5' region of the human platelet-derived growth factor A-chain gene". Hiroshima J. Med. Sci. 42 (1): 47–52. PMID 8486521.
- v
- t
- e
| FGF receptor ligands: | |
|---|---|
| KGF | |
| FGF homologous factors: | |
| hormone-like: FGF15/19 |
Relaxin family
| Insulin and Insulin-like growth factor | |
|---|---|
| Relaxin family peptide hormones |
 | This article on a gene on human chromosome 7 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e